The salt intake of a human in case of either a real survival situation or when living from the provisions of a shoreline by purpose, is of utmost importance to its health. In case too much salt is consumed, there are negative short-term effects on the body, like dehydration, swelling, and kidney damage.


Salt (Sodium chloride NaCl) attracts water and holds it in the human cells. It disrupts osmosis. This causes dehydration and symptoms such as headache, fatigue, and muscle cramps. Swelling in feet, ankles, and legs is also due to retaining body fluid. Negative long-term effects are high blood pressure, osteoporosis, and possibly stomach cancer.
Health organizations recommend that healthy adults limit their sodium intake to 2,300 mg or less per day. Which is about 1 teaspoon of salt. See this link. Too much sodium in the body can be reduced by drinking excessive amounts of fresh water, exercising with drinking lots of water, and eating food with high potassium content.
In a survival situation on a shoreline, there will rarely be excessive amounts of freshwater available. But food with high potassium content is typically seafood. Most finfish have a 4 – 8 times higher potassium content compared to their sodium content. The same goes for crayfish, clams, squid, mussels, and others.
What are now possible salt sources when walking and living from a shoreline? These sources are salt-containing sea breeze, salt in seafood, and by drinking salty water.
How much salt is contained in the sea breeze?
Atmospheric salt from a sea breeze is a mixture of various dissolved salts, like NaCl, KCl, Na2SO4, and various others. For analysis, all of them are commonly converted to ‘equivalent NaCl’ values. See the following link. This, however, does not give accurate figures for sodium, as the ‘equivalent NaCl’ values also include other elements, like potassium, sulfur, magnesia, and calcium. It was found that max. 78% of total salt was converted to ‘equivalent NaCl’.



Besides that inaccuracy, the NaCl content of air heavily depends on wind speeds from sea to land. Up to wind speeds of 14 km/h, all salts are contained in aerosols. Above a wind speed of about 14 km/h, heavy droplets are formed, which fall under gravitational influence.
As an example, at a wind speed of 2 m/s (which corresponds to 7,2 km/h), a wind cone will begin stretching horizontally into the air. At such a wind speed, the sea breeze will contain about 8 microgram salt/m3 of air. Influences of ambient temperatures and humidity are not discussed here in this context.
We therefore can calculate as follows: 8 microgram salt/m3 air x 0,78 (NaCl equivalents) = 6,24 microgram NaCl/m3 air = 0,00624 mg NaCl/m3 air. 1 g salt contains 400 mg sodium. Therefore: 0,00624 mg NaCl/m3 air = 0,0025 mg sodium/m3 air
A human being needs at full rest about 11 m3 air/day (equals 388 cubic feet/day), which can increase under duress, tension, high ambient temperatures, body temperature, and other factors about three-fold. Let’s assume such a bad case of using 30 m3 air/day. If 1 m3 air contains 0,0025 mg sodium/m3 air, then 30 m3 air over the whole day will contain 0,075 mg sodium in total per day.
The sea breeze is therefore not a significant contributor to taking in sodium into the human body.
How much sodium is contained in seafood?
In the following, typical seafood collected or caught on a shoreline are listed. Figures for these food items were sourced from the following link.



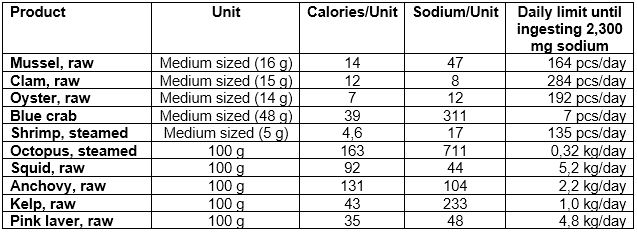
It can be seen that all these typical survival food items on a shoreline – except steamed octopus – are low in sodium and the salt intake is therefore low. And from other sources, we know that their potassium content is between 4 – 8 times higher, which benefits the neutralization of sodium in the blood. Typical survival seafood is therefore not a significant contributor of sodium (respective salt) to the body.
And what is the sodium content when drinking salty water?
There are stories of castaways on an island, who survived long times with a limited water supply. How did they do it? They discovered that freshwater can be diluted with seawater and extended in this way their freshwater sources plus got a healthy mineral content.
Saltwater is 4 times saltier than freshwater. If drinking undiluted saltwater, it acts like a poison and leads to osmosis failures in the cells as mentioned above. However, mixing ¼ parts of seawater with ¾ parts of saltwater will result in an isotonic water ideal for the functioning of the human body. Such isotonic water has a very similar salt content to human blood. However, the amount of isotonic water should be reduced to a maximum of 1 liter (or quart) per day.



Ocean salinity is on average 35 grams of salt per liter of seawater. 1 gram of salt (Sodium chloride) contains 400 mg of sodium. The daily recommended limit of sodium intake is 2,300 mg. Therefore, not more than about 6 grams of salt (about. 1 teaspoon) should ideally be ingested per day. When mixing ¼ of a liter of seawater with ¾ of a liter of freshwater, this will contain abt. 8,75 grams of salt. Which is still acceptable in a survival situation but only for a short period in case of being exposed to other sodium/salt sources.
All-in-all it can be concluded, that in a shoreline survival situation, neither sea breeze nor seafood are significant sodium contributors. It is the ingested seawater, that increases the salt intake, respective sodium level dramatically. Hypertonic (undiluted) seawater on food or direct mouth contact should therefore be avoided as much as possible.
Lessons learned about salt intake in survival situations:
- Although sea breeze tastes salty on the lips, the ingested sodium content is very low
- Typical shore survival seafood is low in sodium but high in potassium
- Isotonic (4 times diluted) seawater can be drunk up to a maximum of 1 liter/day
- Hypertonic (undiluted) seawater should be avoided as much as possible



